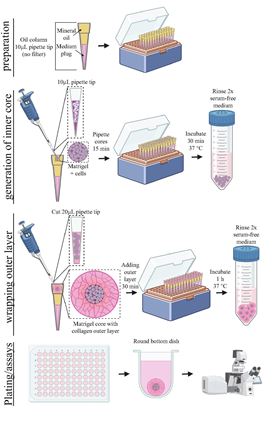
 Schematic of the oil-in-water technology to make uniform, small-volume, two-compartment tumor organoids.
Schematic of the oil-in-water technology to make uniform, small-volume, two-compartment tumor organoids.Cell culture systems known as tumor organoids mimic the physiological microenvironment to enable researchers to maintain and study cancer cells from patients in vitro. Tumor organoids offer cell conditions closer to the physiological environment than traditional 2D or monolayer culture systems but are limited by containing only one extracellular matrix (ECM) compartment instead of the two found in solid tumors (the tumor itself and the surrounding stroma). Now researchers at The Johns Hopkins University and School of Medicine have developed an oil-in-water droplet microtechnology that enables the production of uniform, small-volume organoids with more than one compartment, more accurately resembling the anatomy of tumors [Lee et al., Materials Today (2022), https://doi.org/10.1016/j.mattod.2022.07.006].
“Our organoid culture system uses an oil-in-water droplet technique to establish 3D cultures of cancer cells,” explains one of the authors of the study, Ashleigh J. Crawford. “We have control over the cellular and extracellular content, and the cell-cell and cell-extracellular matrix interactions within the organoids… [giving us] the ability to fine tune the cellular and extracellular tumor microenvironment to match the physiological environment.”
The 3D microenvironment is vitally important to maintaining accurate cell function in vitro. Mimicking these conditions is, therefore, crucial to identifying and studying molecular pathways that drive tumor growth, pinpointing cancer biomarkers, and predicting patient responses to customized drug treatments. The multi-compartment organoid system developed by Denis Wirtz and his colleagues simulates the tumor-stroma interface, allowing the researchers to observe the interactions of multiple cell types while only requiring small volumes of extracellular matrix reagents. The approach enables the investigation of both the proliferation and invasion of cancer cells on a single platform.
The organoids are formed by making small droplets (0.5 mm3) containing cells and biologically accurate extracellular matrix in an oil column and wrapping these ‘cores’ in a larger volume (6 mm3) of another extracellular matrix to mimic the tissue surrounding a tumor. The researchers tested the system with multiple cell types including primary breast and ovarian cancer cells, as well as immortalized cell lines.
“We found that the results of drug screening in this organoid method correlate with clinical data,” says another author, Gabriella C. Russo. “Our system is suitable for establishing tumors, even in cell lines and primary samples notoriously difficult to handle with current techniques.”
The approach will be useful for cell line culture, primary cell culture, and transplantation studies in mice, as they demonstrate, for a variety of cancers, suggest the researchers.